Construction of the oxaphenalene skeletons of mansonone F derivatives through C–H bond functionalization and their evaluation for anti-proliferative activities†
Abstract
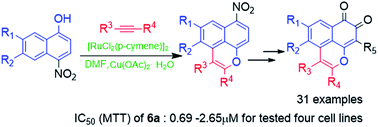
Novel mansonone F derivatives were conveniently synthesized via a key step of Ru(II)-catalyzed C–H functionalization to rapidly construct oxaphenalene skeletons. This synthetic procedure is sufficiently robust and flexible to offer both the generation of diverse mansonone F analogs and the scale-up synthesis of selected compounds. The structural formulas of all products were confirmed and characterized using spectral data. Most of the derivatives exhibited significant cytotoxicity against four tested human tumor cell lines in vitro.


 Please wait while we load your content...
Please wait while we load your content...