Four-component synthesis of polyhydroquinolines under catalyst- and solvent-free conventional heating conditions: mechanistic studies†
Abstract
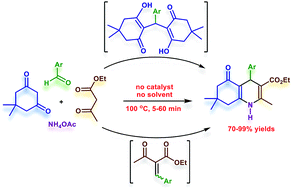
A convenient and environmentally friendly procedure for the synthesis of polyhydroquinolines via a one-pot, four component condensation of different aromatic aldehydes with dimedone, ethyl acetoacetate and ammonium acetate has been developed. Upon heating at 100 °C, the desired products were produced in good to excellent yields with short reaction times under catalyst- and solvent-free conditions. Mechanistic studies indicated that two possible pathways can be accounted for the four-component synthesis of polyhydroquinolines. Unexpectedly, the first involves a nucleophilic attack of a Michael intermediate by an enamine, followed by a retro-aldol-type reaction and a six-electron ring cyclization. The second, which was previously proposed, involves a Michael addition of a Knoevenagel intermediate and an enamine.


 Please wait while we load your content...
Please wait while we load your content...