Ent-abietane diterpenoids and their probable biogenetic precursors from the roots of Euphorbia fischeriana†
Abstract
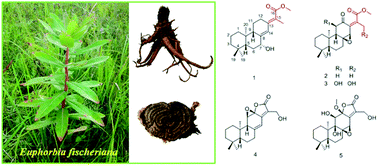
Five new ent-abietane diterpenoids (1–5), along with nine known analogues (6–14), were isolated from the roots of Euphorbia fischeriana. Their structures and absolute configurations were determined by the analysis of extensive spectroscopic data (UV, IR, HRESIMS, NMR) and the comparison of the experimental and calculated electronic circular dichroism (ECD). Among the isolates, it's notable that the ent-abietane diterpenoids bearing an additional five-membered lactone ring might be derived from the proposed precursors of fischeriabietane B (2) and C (3), and their plausible biosynthetic relationship was detailed discussed. All the isolates were evaluated for cytotoxicity against three human cancer cell lines (Panc-28, Bel-7402, and HT-29), 2 and 3 exhibited moderate cytotoxicity.


 Please wait while we load your content...
Please wait while we load your content...