Late stage iodination of biologically active agents using a one-pot process from aryl amines†
Abstract
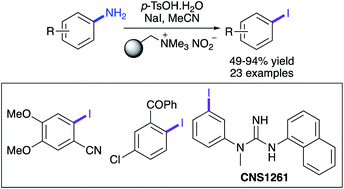
A simple and effective one-pot tandem procedure that generates aryl iodides from readily available aryl amines via stable diazonium salts has been developed. The operationally simple procedure and mild conditions allow late-stage iodination of a wide range of aryl compounds bearing various functional groups and substitution patterns. A novel synthetic strategy involving the preparation of nitroaryl compounds followed by a chemoselective tin(II) dichloride reduction and the use of the one-pot diazotisation–iodination transformation was also developed. The general applicability of this approach was demonstrated with the preparation of a number of medicinally important compounds including CNS1261, a SPECT imaging agent of the N-methyl-D-aspartate (NMDA) receptor and IBOX, a compound used to detect amyloid plaques in the brain.


 Please wait while we load your content...
Please wait while we load your content...