Aromatic poly(ether ether ketone)s capable of crosslinking via UV irradiation to improve gas separation performance
Abstract
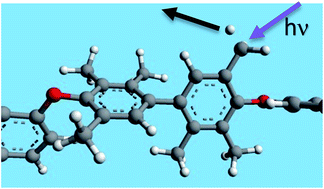
The effect of UV-crosslinking on the gas transport properties of two poly(ether ether ketone)s derived from difluorobenzophenone and two bisphenol derivatives, with four (TMBP-DFB) or six (HMBP-DFB) methyl groups, has been studied. The crosslinking reaction was conducted on dense membranes, using polychromatic light, with wavelengths higher than 350 nm, at room temperature and in presence of air. Both polymers were able to produce crosslinked membranes, with gel fractions close to 75%, but a shorter irradiation time was required for HMBP-DFB. A DFT quantum mechanical study has stated that HMBP-DFB radical formation is much easier than for TMBP-DFB, which would support the fastest kinetics of the crosslinking process for HMBP-DFB. The crosslinked membranes have shown greatly improved gas transport properties, especially for the O2/N2 gas pair, where the Robeson upper bound line of 1991 was clearly surpassed. The improvement in selectivity has been ascribed to the better molecular-sieving characteristics of crosslinked membranes.


 Please wait while we load your content...
Please wait while we load your content...