Understanding the regioselectivity of Michael addition reactions to asymmetric divinylic compounds†
Abstract
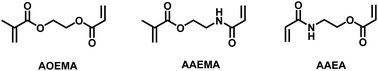
In the present paper, we describe the synthesis of novel monomers prepared by regioselective Michael addition to asymmetric divinylic compounds. This chemoselectivity was experimentally studied employing different reaction conditions and theoretically calculated using chemical global and local descriptors. The global reactivity data show that incoming nucleophilic secondary amines preferentially attack the acrylic derived units acrylate and acrylamide, while deactivated methacrylate and N-vinyl-pyrrolidone require harder reaction conditions, which leads to the formation of by-products. Moreover, it is demonstrated that the presence of two vinyl units within a studied divinylic agent leads to a significant increase in its global reactivity parameters. Besides, the local reactivity parameters of asymmetric divinyl compounds show a preference for an attack at the Cβ of activated units compared to the Cβ center of deactivated units. Based on these results, asymmetric divinyl compounds are very interesting starting materials for the preparation of new functionalized monomers.


 Please wait while we load your content...
Please wait while we load your content...