Synthesis of poly(n-hexyl methacrylate)-b-poly(methyl methacrylate) via anionic polymerization with t-BuOK as the initiator at ambient temperature
Abstract
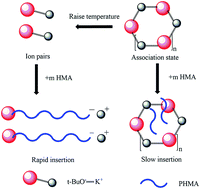
To break through the industrial development bottlenecks of anionic polymerization in the field of polar monomers, poly n-hexyl methacrylate (PHMA) was prepared at 0 °C, 30 °C, and 60 °C with potassium tert-butoxide (t-BuOK) as the initiator in tetrahydrofuran (THF) and the conversions were almost complete. Gel permeation chromatography (GPC) analysis of poly n-hexyl methacrylate indicated that t-BuOK existed in numerous states when dissolved in THF, including different types of association and ion-pairs. A series of block copolymers of n-hexyl methacrylate (n-HMA) and methyl methacrylate (MMA), PMMA-b-PHMA (Mn 8200–16 200) and PHMA-b-PMMA (Mn 19 100–9500) were synthesized at 0 °C, and conversions were close to 100%. The results of dynamic mechanical analysis (DMA) indicated that there were two glass transition temperatures, −10 to 0 °C for the PHMA block and 100 to 110 °C for the PMMA block. Furthermore, the competing behaviors of n-HMA and MMA in copolymerization were investigated by one-shot feeding of two types of monomers at 0 °C, 30 °C, and 60 °C. The kinetics curves and 13C-NMR analysis of the copolymers indicated that the polymerization of n-HMA in the copolymerization was significantly dominant over that of MMA at set temperatures. The results in this study provided the possibility to realize anionic polymerization of methacrylate esters on a commercial scale.


 Please wait while we load your content...
Please wait while we load your content...