Oxygenated graphene quantum dots (GQDs) synthesized using laser ablation for long-term real-time tracking and imaging†
Abstract
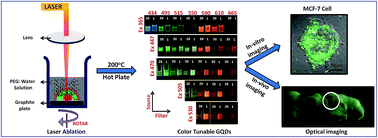
Fluorescence probes are essential for in vivo molecular imaging as well as cell tracking applications. Probes that emit in the far red wavelength penetrate better through biological tissue and the autofluorescence background is reduced at higher wavelengths, making such probes highly desirable. We report the application of Graphene Quantum Dots (GQDs) as efficient fluorescence probes for single cell tracking using high-resolution confocal microscopy as well as a potential in vivo fluorescent imaging agent. High-quality water soluble GQDs were synthesized by ablating highly oriented pyrolytic graphite (HOPG) in liquid using a nanosecond pulsed laser. Refluxing GQDs at 200 °C for 20 minutes and 1 hour produces excitation independent broad emission peaking at 600 nm and excitation dependent color-tunable emission, respectively. These emission properties can be attributed to the functional groups such as carboxyl and hydroxyl groups on the surface or edges. MTT assays with the breast cancer (MCF-7) cell line suggest that 20 min and 1 h GQDs had 80% viability post incubation with concentrations as high as 2 mg mL−1. The paper also explores the molecular tracking functionality of GQDs. Fluorescence microscopy showed that MCF-7 cells incubated for up to 48 hours with GQDs, internalized the GQDs, by caveolae-mediated endocytosis without any targeting moiety. In addition, high-resolution Z stacking and 3D confocal microscopy of a single live MCF-7 cell confirm successful uptake of GQDs into the cytoplasm by endocytosis. Fluorescence imaging of GQDs loaded in polyacrylamide gel and subcutaneously implanted near the thoracic region of an euthanized mice was done to explore the feasibility of in vivo imaging using GQDs. Deep red fluorescence (610 nm emission filter) was observed from the implanted region with relatively low autofluorescence background.


 Please wait while we load your content...
Please wait while we load your content...