Biochemical, thermodynamic and structural studies of recombinant homotetrameric adenylosuccinate lyase from Leishmania braziliensis†
Abstract
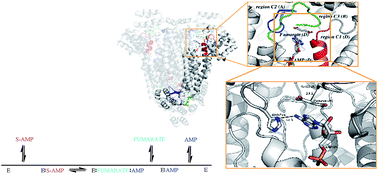
Adenylosuccinate lyase (ASL) is involved in both de novo and salvage pathways of purine biosynthesis. ASL belongs to the argininosuccinate lyase/fumarase C superfamily of enzymes which share a general acid–base catalytic mechanism with β-elimination of fumarate as the common product. Cloning, expression, and a method to obtain homogeneous recombinant ASL from Leishmania braziliensis (LbASL) are described. Mass spectrometry analysis of recombinant LbASL, oligomeric state determination and multiple sequence alignment are presented. Steady-state kinetics of LbASL showed a Michaelis–Menten pattern. Isothermal titration calorimetry binding assays suggested that LbASL follows a Uni-Bi ordered kinetic mechanism, in which release of fumarate is followed by AMP to yield free enzyme. Initial velocity data for the reverse reaction and the Haldane relationship allowed calculation of an unfavorable equilibrium constant for the LbASL-catalyzed chemical reaction. The activation energy and thermodynamic activation parameters were estimated. Solvent kinetic isotope effects V/K and V suggest a modest contribution of solvent proton transference during the rate-limiting step of the reaction. Proton inventory data show that the modest normal effect on V arises from a single protonic site, and the transition state fractionation factor value of 0.74 suggests participation of solvent proton transfer in transition-state vibrations perpendicular to the reaction coordinate. pH-rate profiles for kcat and kcat/KM suggested amino acid residues involved in, respectively, catalysis and substrate binding. A model of LbASL was built to provide a structural basis for the experimental data. A better understanding of the mode of action of LbASL is useful for the rational design of antileishmaniasis agents.


 Please wait while we load your content...
Please wait while we load your content...