Novel type ketone-substituted metallophthalocyanines: synthesis, spectral, structural, computational and anticancer studies
Abstract
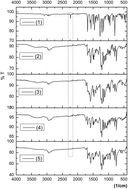
This work reports on the synthesis and characterization of phthalocyanines (M = Cu(II) (2), Zn(II) (3) In(III) (4) and Co(II) (5)) peripherally tetra-substituted with 1-(4-hydroxyphenyl)propan-1-one. Confirmation of the synthesized phthalocyanine structures are performed with a combination of elemental analysis, FTIR, 1H-NMR, 13C-NMR, UV-vis and MALDI-MS SEM and spectral data. Their aggregation properties were examined in THF by UV-vis. Spectral and photophysical (fluorescence quantum yield) properties of complexes (2–4) were reported in THF (tetrahydrofuran). These results suggest that the metal in the core of the phthalocyanine plays an important role in the fluorescence quantum yields ΦF of the synthesized complexes (2–4). Also, the anticancer activities of complexes were studied on MCF-7, MG63, and L929 cell lines. Finally, all synthesized phthalocyanines were investigated by quantum chemical studies. Chemical reactivity parameters such as EHOMO, ELUMO, ΔE (HOMO–LUMO energy gap) were calculated by Gaussian software.


 Please wait while we load your content...
Please wait while we load your content...