Electrochromism and electrochemical properties of complexes of transition metal ions with benzimidazole-based ligand†
Abstract
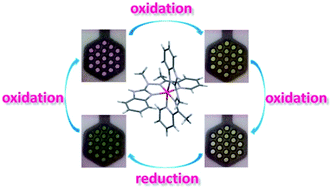
Six new mononuclear complexes of transition metal ions (1–6) with (E)-2-(1-methyl-2-(1-(pyridin-2-yl)ethylidene)hydrazinyl)-1H-benzoimidazole L were synthesized and characterized by spectroscopic methods as well as X-ray diffraction analysis. Structures of these complexes can be divided into three groups depending on the coordination number and environment of the metallic center: the first one of general formula [ML2]X2, the second one - [MLX3] and the third one - [MLX2], where X is anion or solvent molecule. Electrochemical and electrochromic properties of complexes were investigated. Complexes Fe(II) 1, Cu(II) 2–4 and Co(II) 5 were found to be electroactive and their colors depend on the oxidation state of metal center and ligand molecule. Fe(II) complex 1 exhibits purple color in the neutral state and its color changes to yellow after Fe(II) → Fe(III) oxidation, followed by pale-yellow after further oxidation of ligand molecule and to green when the metal center is reduced to Fe(I). The original color can be restored after electrochemical oxidation Fe(I) to Fe(II). It was found that this complex exhibits high color stability in solution during multiple oxidation/reduction cycles. This complex can be therefore regarded as very interesting material for the construction of multielectrochromic devices.


 Please wait while we load your content...
Please wait while we load your content...