An ionic liquid–organic solvent biphasic system for efficient production of 5-hydroxymethylfurfural from carbohydrates at high concentrations
Abstract
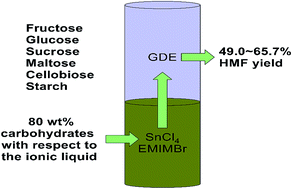
5-Hydroxymethylfurfural (HMF) is an important intermediate in the utilization of lignocellulosic biomass. Ionic liquid 1-ethyl-3-methylimidazolium bromide (EMIMBr) containing a dissolved SnCl4 as catalyst was found to be capable to convert carbohydrates into HMF, as an alternative to the chromium-based catalytic system. Based on this, a biphasic system consisting of glycol dimethyl ether or dimethyl carbonate as the extraction phase, and EMIMBr/SnCl4 in combination with partial dissolved organic solvent as the reaction phase was developed for the production of HMF from carbohydrates (80 wt% with respect to the ionic liquid) at high concentrations. The biphasic system could obtain HMF yields (49.0–65.7%) comparable to or slightly higher than the monophasic system. The biphasic system could not only considerably displace ionic liquids with organic solvents, but also promote the separation of HMF from the reaction system owing to the excellent extraction ability and low boiling point of the organic solvents.


 Please wait while we load your content...
Please wait while we load your content...