Removal of Cu(ii) from aqueous solution using Fe3O4–alginate modified biochar microspheres
Abstract
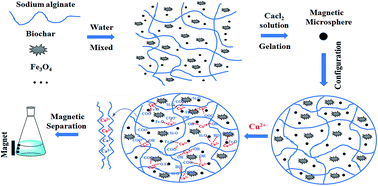
Magnetic microspheres (MM) were prepared using calcium alginate (CA) encapsulated biochar (BC) and Fe3O4 as a high-performance green absorbent for Cu(II) removal from aqueous solution. The effects of the initial Cu(II) concentration, initial pH value of the Cu(II) solution and equilibrium contact time were investigated. The results revealed that the adsorption capacity of MM was approximately 3 times higher than that of the magnetic biochar (MBC). The adsorption of Cu(II) by the MM was in good agreement with pseudo-second-order kinetics. The overall orders for fitting the isotherm models are Langmuir > Freundlich for Cu(II). Mechanism studies showed that coordination and ion exchange were the major mechanism for Cu(II) removal by the MM. Moreover, the presence of the magnetic particles in the microspheres allowed easy separation of the material from aqueous solutions. This study provides an effective method for biochar modification.


 Please wait while we load your content...
Please wait while we load your content...