Thiocarbamoyl in synthesis of the absorbent bis-mono and dimethine cyanine dyes incorporating porphyrin, thiazoles, thiazolones, thiadiazoles, pyrazoles and pyrazolones†
Abstract
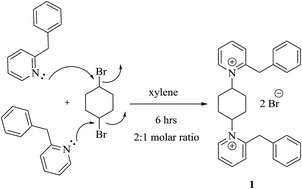
In this study, we have introduced an outlook dealing with synthetic heteroarenium-substituted pyridines 1 that are suitable as starting materials for the synthesis of a potential new thiocarbamoyl derivative 3 that may be of value in preparative organic and biological chemistry. Herein, a new series of twelve bis-mono and dimethine cyanine dyes incorporating different heterocyclic compounds were designed by efficient and simple reaction of compound 3 with various organic reagents to overcome the obstacles of energetic reactions, because they took long times. Moreover, the electronic behaviors of these compounds were studied because of their significant activity in many medical and other fields. The photosensitizers were elucidated by spectral and analytical analyses.


 Please wait while we load your content...
Please wait while we load your content...