WO3–EDA hybrid nanoplates and nanowires: synthesis, characterization, formation mechanism and thermal decomposition†
Abstract
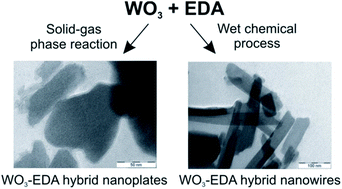
Previously the WO3–EDA hybrid material was obtained only from solvothermal reactions. In this study this hybrid was prepared by two novel methods: a solid–gas phase heterogeneous reaction, and a wet chemical process. In the case of the solid–gas phase reaction the effects of the composition, crystal structure, and the particle size of the WO3 powder and the presence of H2O vapor were studied, while in the case of the wet chemical process the effect of the solvent was investigated. The structure, composition, morphology and thermal decomposition of the as-prepared WO3–EDA hybrid were investigated by XRD, FTIR, solid-state NMR, elemental analysis, SEM, TEM and TG/DTA-MS measurements. In addition, its catalytic activity was tested in a Knoevenagel condensation model reaction. Based on the results the WO3–EDA empiric formula was proposed to replace the current WOx–EDA formula. From the solid–gas phase reaction WO3–EDA nanoplates were obtained for the first time. Furthermore, a new formation mechanism was proposed for the solid–gas phase formation of this hybrid material. The thermal decomposition of the hybrid resulted m-WO3 in air, and an amorphous tungsten oxide phase in nitrogen. During annealing, the evolved EDA transformed into a series of heterocyclic aromatic compounds in both atmospheres. The as-prepared hybrids had the same catalytic properties as the hybrids obtained previously from the solvothermal reactions.


 Please wait while we load your content...
Please wait while we load your content...