Nitrones and nucleobase-containing spiro-isoxazolidines derived from isatin and indanone: solvent-free microwave-assisted stereoselective synthesis and theoretical calculations†
Abstract
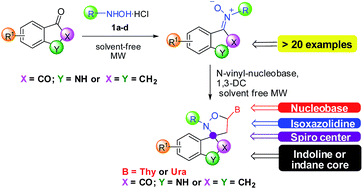
The spiro-oxindoles have found wide application because of their antiviral properties. However, in the literature few examples of synthesis of their precursors, oxindole-nitrones, are reported. In this paper, we initially present a rapid and efficient synthetic approach to ketonitrones by solvent-free microwave-assisted reaction between isatin or indanone derivatives and various hydroxylamines. The synthetic protocol is facile, clean, fast, high-yielding and stereoselective. Then, we explored the possibility to synthesize nucleobase-containing spiro-isoxazolidines with isatin and indanone nuclei by solvent-free MW-assisted 1,3-dipolar cycloaddition, obtaining good results in yields (74–85%), and regio- and diastereoselectivity. Theoretical calculations were done to analyze the difference of reactivity of isatin and indanone derivatives with hydroxylamines.


 Please wait while we load your content...
Please wait while we load your content...