Design, synthesis, and herbicidal activity of pyrazole benzophenone derivatives†
Abstract
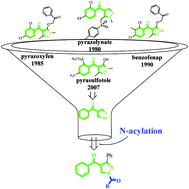
4-Hydroxyphenylpyruvate dioxygenase is one of the most promising targets for herbicide discovery. A series of 1-acyl-3-phenyl-pyrazol benzophenones were designed and synthesized using 1,3-diphenylpropane-1,3-dione and dimethylformamide dimethylacetal as the starting materials. All of the compounds were characterized by IR, 1H NMR, 13C NMR, and HRMS. The configuration of 5f was determined by X-ray crystallography. The bioassay studies indicated that most of these derivatives exhibited herbicidal activity at least to a certain degree, in which compounds 5n and 5o displayed good herbicidal activity at a dosage of 0.05 mmol m−2, which were more potent than pyrazoxyfen against barnyard grass. In addition, compound 5o was also proved to be safer for maize than pyrazoxyfen. The binding free energy of compound 5o with HPPD was relatively low, and that agreed with the results of bioassay activity research. Therefore, compound 5o might be the lead compound for designing new HPPD inhibitors.


 Please wait while we load your content...
Please wait while we load your content...