Synthesis, crystal structure, photophysical property and metal ion-binding behavior of a cyclometalated platinum(ii) terpyridylacetylide with efficient π-conjugation degree†
Abstract
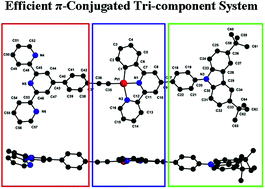
A cyclometalated Pt(II) acetylide derivative bearing a carbazole donor and a terpyridine (TPY) receptor has been successfully synthesized and characterized. X-ray crystallography shows its monoclinic crystal system with the P2(1)/c space group and the three-component co-planarity molecular structure. This efficient π-conjugation system displays an intense low-energy absorption band (ε = 1.51 × 104 dm3 mol−1 cm−1) at λmax = 442 nm and a strong phosphorescence emission (Φ = 0.045) at λmax = 596 nm in an air-saturated CH2Cl2 solution at room temperature, resulting from the dπ(Pt) → π*(C^N^N) metal-to-ligand charge transfer (MLCT) mixing with the π(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–Ar) → π*(C^N^N) ligand-to-ligand charge transfer (LLCT) transition. With the introduction of the TPY receptor, this complex possesses ca. 1.6-fold luminescence-enhancing response for Zn2+ and Cd2+ but dramatic luminescence quenching response toward other common transition metal cations. Consecutive titrations exhibit that the added metal ions bond to its free TPY receptor with 1 : 2 stoichiometry to form the heterotrinuclear (Pt–M–Pt) complex. The Fe2+-quenched and Zn2+-enhanced luminescence behaviors with the maximum PET efficiency of 92.4% and 62.7%, respectively, strongly suggest the presence of the intra-molecular energy transfer between the [M(TPY)2]2+ core and the terminal phosphorescent Pt(II) complex units.
C–Ar) → π*(C^N^N) ligand-to-ligand charge transfer (LLCT) transition. With the introduction of the TPY receptor, this complex possesses ca. 1.6-fold luminescence-enhancing response for Zn2+ and Cd2+ but dramatic luminescence quenching response toward other common transition metal cations. Consecutive titrations exhibit that the added metal ions bond to its free TPY receptor with 1 : 2 stoichiometry to form the heterotrinuclear (Pt–M–Pt) complex. The Fe2+-quenched and Zn2+-enhanced luminescence behaviors with the maximum PET efficiency of 92.4% and 62.7%, respectively, strongly suggest the presence of the intra-molecular energy transfer between the [M(TPY)2]2+ core and the terminal phosphorescent Pt(II) complex units.


 Please wait while we load your content...
Please wait while we load your content...