Ring-locking strategy facilitating determination of absolute optical purity of 2-amino-1-butanol by reverse-phase high-performance liquid chromatography†
Abstract
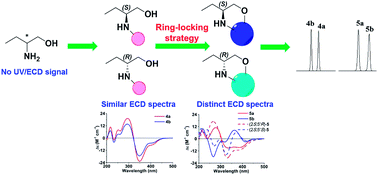
A concise and efficient reverse-phase high-performance liquid chromatography (RPLC) method has been established for absolute optical purity assay of 2-amino-1-butanol, which is an important synthetic intermediate of various drugs. Its enantiomers were derivatized with methyl (5-fluoro-2,4-dinitro-phenyl)-(S)-prolinate, which could be regarded as a structural variant of Marfey's reagent. The resultant diastereomers were further cyclized using bis(trichloromethyl)carbonate to form oxazolidin-2-one ring, leading to a distinct difference in their electronic circular dichroism (ECD) spectra. Time-dependent density functional theory (TDDFT) was then applied to simulate theoretical ECD spectra for unambiguous absolute configuration assignment of cyclized products without putative samples. Both pairs of diastereomers could be separated on a reverse-phase C18 column using a mobile phase of methanol or acetonitrile and water with UV detection at 336 nm. The tandem reactions could be integrated into one-pot synthesis with high yield. Thus, exact absolute optical purity of 2-amino-1-butanol could be directly determined by monitoring the peak ratios of these diastereomers. This method is well worth extending to the absolute optical purity assessment of other chiral amino alcohols.


 Please wait while we load your content...
Please wait while we load your content...