A short synthesis of 7-amino alkoxy homoisoflavonoides†
Abstract
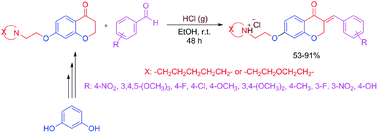
The synthesis of novel derivatives of homoisoflavonoids as potentially interesting medicinally important heterocycles in an efficient catalytic two step route is introduced. In the first step, 7-aminoalkoxychromane-4-ones are synthesized via reaction between 7-hydroxychroman-4-one and aminoethylchlorides in the presence of potassium carbonate as a Brønsted base catalyst. In the next step, obtained 7-aminoalkoxychromane-4-ones are reacted with a wide range of arylaldehydes in the presence of hydrochloric acid as Brønsted acid catalyst to obtain homoisoflavonoids. Target products are medicinally very important heterocycles, because their analogs show cytotoxic activities towards human cancer cell lines.


 Please wait while we load your content...
Please wait while we load your content...