Improvement of the amphiphilic properties of a dialkyl phosphate by creation of a protic ionic liquid-like surfactant†
Abstract
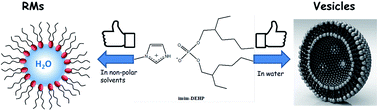
In the present work we have synthesized, via acid–base reaction between bis-(2-ethylhexyl)phosphoric acid (HDEHP) and 1-methylimidazole, a new protic ionic liquid: 1-methylimidazolium bis-(2-ethylhexyl) phosphate (imim-DEHP) with amphiphilic character. The ability of imim-DEHP to create RMs in different nonpolar solvents (n-heptane, benzene and toluene) and spontaneously unilamellar vesicles in water was evaluated by different techniques such as dynamic (DLS) and static (SLS) light scattering and transmission electron microscopy (TEM). The results obtained were compared with the properties of sodium bis-(2-ethylhexyl) phosphate (Na-DEHP) in order to elucidate how the surfactant chemical structure affects the organized media generated. Our results show that imim-DEHP forms RMs in aromatic and aliphatic nonpolar solvents and allows the dissolution of a considerable amount of water in both systems. Additionally, imim-DEHP has the capacity to create spontaneously unilamellar vesicles. Interestingly, imim-DEHP shows better characteristics as a surfactant than Na-DEHP. In this sense, we consider that the present work introduces imim-DEHP as a very promising surfactant for future applications as nanoreactors and in nanotechnology.


 Please wait while we load your content...
Please wait while we load your content...