A novel fluorescence turn-on probe for the selective detection of thiophenols by caged benzooxazolidinoindocyanine†
Abstract
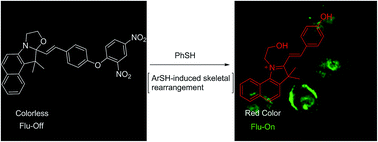
Based on a strategy involving 2,4-dinitrophenyl ether and functionalized caged benzooxazolidinoindocyanine, a fluorescence turn-on probe for the selective detection of thiophenols was developed. Using the chemical properties of thiophenols, which are able to induce aromatic nucleophilic substitution (SNAr), and the principle of chemical-reaction-induced skeletal rearrangement, the probe (PBO) exhibits excellent selectivity for thiophenols with a detection limit of 7 nM. This probe features dramatic color changes and a significant fluorescence turn-on response (120 fold) after being treated with thiophenol. The applications of this new probe were demonstrated by the detection of thiophenol in real water samples and by fluorescence imaging of thiophenol in BEL-7402 cells.


 Please wait while we load your content...
Please wait while we load your content...