Synthesis, cytotoxic activity and drug combination study of tertiary amine derivatives of 2′,4′-dihydroxyl-6′-methoxyl-3′,5′-dimethylchalcone†
Abstract
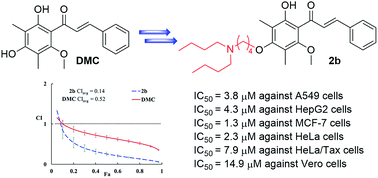
In an effort to develop more water soluble anticancer drugs based on 2′,4′-dihydroxyl-6′-methoxyl-3′,5′-dimethylchalcone (DMC), a group of DMC derivatives bearing polar and ionizable tertiary amine functionalities were synthesized. Cell based MTT assay resulted in the discovery of one compound (2b) which shows a broad spectrum of cytotoxic activity (IC50 < 5 μM against all tested sensitive cancer cells) and moderate selectivity between normal and tumor cells. Further drug combination study uncovered that all of the semi-synthetic DMC derivatives (2a–2f) acted synergistically with anticancer drug Taxol® to achieve improved anti-proliferative efficacy against drug resistant HeLa/Tax cells. The combinations of Taxol® with each of the two most effective compounds (2b and 2d) displayed weighted average Combination Index (CI) values of 0.14 and 0.15, respectively, and significantly reduced the dosage of Taxol® while maintaining the same level of efficacy. Our findings suggested that the introduction of a polar and ionizable tertiary amine functionality into the 4′-OH of DMC is a feasible way to improve its aqueous solubility, anticancer activity, and cancer selectivity.


 Please wait while we load your content...
Please wait while we load your content...