Gaseous cyclohexanone catalytic oxidation by a self-assembled Pt/γ-Al2O3 catalyst: process optimization, mechanistic study, and kinetic analysis†
Abstract
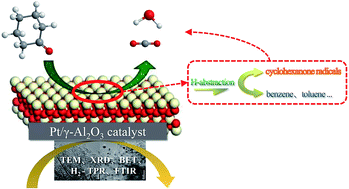
γ-Al2O3 nanocatalysts with a Pt loading of 0.6–1.0% were prepared successfully via a self-assembly method to be used in the catalytic oxidation of cyclohexanone in a fixed-bed reactor. The nanocatalysts were characterized by the Brunauer–Emmett–Teller method, X-ray diffraction, transmission electron microscopy, Fourier-transform infrared spectroscopy, and temperature programmed reduction-H2 to correlate their activity with their physiochemical properties. Based on these analyses, the catalytic oxidation efficiency for cyclohexanone and the optimal catalytic temperature of 1.0% Pt/γ-Al2O3 were the best among all the nanocatalysts. A novel response surface methodology (RSM) method was employed to evaluate the interactions of the gaseous cyclohexanone concentration (500–4000 mg m−3), the gas hourly space velocity (GHSV, 5000–20 000 h−1), and relative humidity (RH 10–70%) on the catalytic oxidation under normal atmospheric pressure and a catalytic temperature of 235 °C. When the initial catalyst concentration, GSHV, and RH were 2000 mg m−3, 12 000 h−1, and 50%, respectively, the efficiency was 86.5%, nearly equal to that (89.6%) predicted by the RSM model. Intermediate generation, CO2 production, and variations in the cyclohexanone oxidation route were evaluated under the optimal process parameters. During catalytic oxidation, nearly all the cyclohexanone was converted to CO2 and H2O, and the mineralization rate was 80% under a RH of 50%. A kinetic model was proposed to describe the interaction effects between the partial pressure and catalytic temperature on the conversion of cyclohexanone. It was found that the predicted values by the model fit well with the experimental values, with an R2 of 0.99.


 Please wait while we load your content...
Please wait while we load your content...