Exploring sesquiterpene alkaloid-like scaffolds via Beckmann-transannular remodelling of beta-caryophyllene†
Abstract
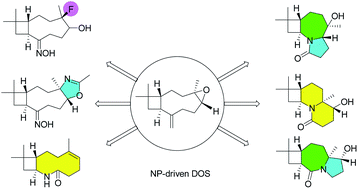
High-throughput screening (HTS) is the dominant approach to identify lead compounds in drug development. However, most compound-screening collections provide little structural or stereochemical complexity, which do not offer enough diversity to merit the modulation of many drug targets. Here we describe a facile strategy for the creation of diverse compounds with high structural and stereochemical complexity using readily available natural products, β-caryophyllene, as a synthetic starting point. Our findings demonstrate that a cascaded Beckmann-transannular protocol transforms macrocyclic natural products into poly-heterocyclic unnatural skeletal types. These compounds are significantly more complex and diverse than those in standard screening collections.


 Please wait while we load your content...
Please wait while we load your content...