Synthesis of 2′-O-monohaloethoxymethyl-modified RNAs and their duplex formation ability†
Abstract
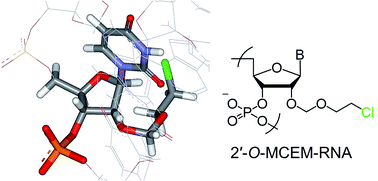
We synthesized 2′-O-monohaloethoxymethyl-modified RNAs and evaluated their duplex formation ability. The effects of 2-chloroethoxymethyl (MCEM) and 2-fluoroethoxymethyl groups on the RNA/RNA duplex stability was found to depend on both base sequences and halogen atoms. Only the 2′-O-MCEM-rU12/rA12 duplex was found to be significantly more stable than the unmodified duplex. In this study, it is proposed through UV melting analyses, isothermal titration calorimetry measurements, and molecular mechanics calculations that this stabilization might result from enthalpic stabilization due to interactions between the MCEM groups and nucleobases in the complementary strand.


 Please wait while we load your content...
Please wait while we load your content...