Rapid production of Pd nanoparticle by a marine electrochemically active bacterium Shewanella sp. CNZ-1 and its catalytic performance on 4-nitrophenol reduction
Abstract
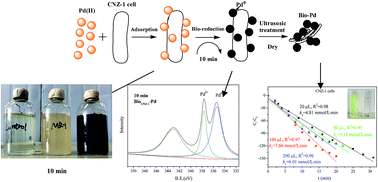
Microbial recovery of Pd through Pd(II) reduction is emerging as a clean alternative to traditional physical and chemical reclaiming treatments. Shewanella species are proven to be capable of excellent metal reduction properties and have thus drawn great attention for Pd(II) reduction. So far, however, only Shewanella oneidensis MR-1 has been explored for Pd nanoparticles (Pd-NPs) production. In this study, we report a marine electrochemically active bacterium Shewanella sp. CNZ-1 with the ability for Pd-NPs generation in the presence of sodium lactate as an electron donor. SEM-EDX, TEM, FTIR, XRD and XPS analyses were performed to analyze the Pd(II) reduction process and products. The results showed that Shewanella sp. CNZ-1 was more efficient than Shewanella oneidensis MR-1 in reducing Pd(II) under current conditions. In addition, Pd(II) reduction by CNZ-1 was inhibited by low pH value (3–6) and high concentration of NaCl (3–9%), whereas Pd(II) adsorption by CNZ-1 was unaffected by these factors. Further study showed that hydrogenase and some amides were involved in Pd(II) reduction by CNZ-1. Besides, Pd-NPs produced by CNZ-1 cells (bioCNZ-1-Pd-NPs) seemed more effective than those produced by MR-1 cells (bioMR-1-Pd-NPs) in mediating 4-nitrophenol (4-NP) reduction by NaBH4. For the bioCNZ-1-Pd-NPs supplemented system, the k1 (zero-order rate constant) values were 2.49-, 2.76- and 1.91-fold of the bioMR-1-Pd-NPs supplemented system when the concentrations of NPs were 33 mg L−1, 83 mg L−1 and 167 mg L−1, respectively. The Pd recovery process by CNZ-1 is simple, efficient and environmentally friendly, and this biotechnology has potential practical application in treating mining wastewater.


 Please wait while we load your content...
Please wait while we load your content...