Solvent-free and melt aerobic oxidation of benzyl alcohols using Pd/Cu2(BDC)2DABCO–MOF prepared by one-step and through reduction by dimethylformamide†
Abstract
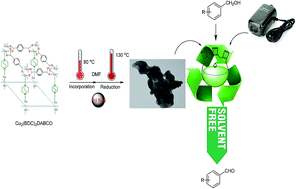
In this paper we report an efficient method for the aerobic oxidation of benzyl alcohols to aldehydes by supported palladium nanoparticles in a nanoporous metal–organic framework Cu2(BDC)2(DABCO) (BDC = 1,4-benzenedicarboxylate, DABCO = 1,4-diazabicyclo[2.2.2]octane) under a temperature control program and through reduction by dimethylformamide. The Pd-NPs/Cu2(BDC)2(DABCO) offered higher catalytic activity and recyclability than reported in literature. The supported Pd nanoparticles (PdNPs/Cu2(BDC)2(DABCO)) were characterized by X-ray diffraction, BET analysis, field emission scanning electron microscopy, transmission electron microscopy, inductively coupled plasma atomic emission spectroscopy and X-ray photoelectron spectroscopy (XPS).


 Please wait while we load your content...
Please wait while we load your content...