Deciphering chemical bonding in BnHn2− (n = 2–17): flexible multicenter bonding
Abstract
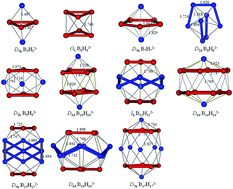
It is well known that closo-borane dianions BnHn2− are stable aromatic cages and possess n + 1 valence electron pairs, in accordance with Wade's rules. However, the electronic structures of closo-BnHn2− cannot be explained by a Lewis structure because of their electron-deficient character, while recent theoretical and experimental studies reveal that multicenter bonding is a key part in their electronic structures. In this work, flexible multicenter bonding of BnHn2− (n = 2–17) is studied using the adaptive natural density partitioning (AdNDP) method in order to get insight into their stability and aromaticity. The large HOMO–LUMO gaps and negative NICS values indicate their close-shell electronic structure. Further chemical bonding analysis shows that all 2n + 2 delocalized valence electrons in closo-BnHn2− are involved in various delocalized σ and π multicenter bonding systems on their cage surface, well matched with different symmetric configurations. There are five types of multicenter bonds, including an open 3c–2e BBB bond, closed 3c–2e BBB bond, 4c–2e bond, 8c–2e bond and a fully delocalized bond, which are delocalized on a B3 broken-line, B3 triangle, B4 rhombus, B8 double-ring and the whole surface of the boron cage, respectively. Our work reveals the flexibility of multicenter bonding in diversified closo-BnHn2− clusters, giving new insights into the bonding nature of BnHn2−.


 Please wait while we load your content...
Please wait while we load your content...