Hemin-bound cysteinyl bolaamphiphile self-assembly as a horseradish peroxidase-mimetic catalyst
Abstract
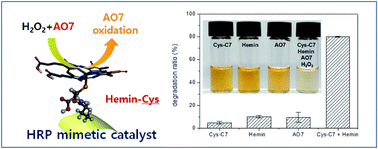
Horseradish peroxidase (HRP) is an important oxidative enzyme with a heme cofactor whose Fe centre acts as the active site. Its catalytic activity is expressed upon association with the heme cofactor through coordination between Fe and histidine imidazole. In this study, a HRP-mimetic catalyst was prepared by binding hemin to the self-assembled suprastructure of cysteinyl bolaamphiphiles through an Fe–thiol bond, and its oxidative catalytic activity was investigated. The cysteinyl bolaamphiphile self-assembly is rich with surface-exposed cysteine thiols, which served as binding sites. The activity of the prepared HRP-mimetic catalyst was pH-dependent and increased with increasing temperature, with an activation energy of 31.7 kJ mol−1. The kinetic parameters obtained by varying the substrate concentrations suggested a ping-pong mechanism where H2O2 and substrate bind sequentially to the active centre. The cysteinyl bolaamphiphile self-assembly provides a biomimetic support on which various metallic cofactors can bind to induce biochemical activity.


 Please wait while we load your content...
Please wait while we load your content...