Palladium-catalyzed synthesis of quinolines from allyl alcohols and anilines†
Abstract
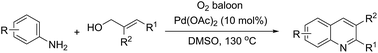
A process for quinoline synthesis through palladium-catalyzed oxidative cyclization of aryl allyl alcohols and anilines is described. This process works in the absence of acid, base and any other additive and has a broad substrate scope, tolerating electron-withdrawing groups such as nitryl, trifluoromethyl and so on. A series of quinolines are prepared in satisfactory yields.


 Please wait while we load your content...
Please wait while we load your content...