Prepared MnO2 with different crystal forms as electrode materials for supercapacitors: experimental research from hydrothermal crystallization process to electrochemical performances
Abstract
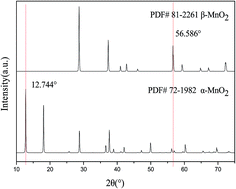
The aim of this study was to prepare manganese dioxide with different crystal forms through hydrothermal treatment of MnSO4·H2O–KMnO4 precursors at various precursor ratios, temperatures, time periods, and pH values. The effect of the preparation parameters on the crystal structure and electrochemical performance of MnO2 was also studied. The conditions for the preparation of each crystal of MnO2 were experimentally optimized. The results indicate that the varied parameters affect the crystal form of MnO2; the ratio of the precursor and reaction temperature are the significant factors affecting the crystal form of MnO2, and the reaction time and pH value mainly affect the integrity of crystallization. Moreover, the presence of the cations K+ and NH4+ assists the crystallization of α-MnO2. δ-MnO2 and amorphous MnO2 have higher specific capacitance. The specific capacitance is determined not only by the crystal form, but also by the parameters of hydrothermal treatment.


 Please wait while we load your content...
Please wait while we load your content...