Facile fabrication of lipase to amine functionalized gold nanoparticles to enhance stability and activity†
Abstract
Among various techniques of immobilization, EDC/NHS cross linking is a simple and single step process for covalent coupling between enzymes and nanoparticles. Here we describe immobilization of lipase on amine functionalized gold nanoparticles (AuNPs-NH2) to attain enhanced activity and stability. To achieve a suitable orientation, it is necessary to understand the contribution of different functional groups on the enzyme's surface. Therefore, the crystal structure of lipase was analyzed using a computational method (PyMOL) to find the exposed acidic amino acid residues that can be exploited for conjugation. Confirmation of conjugation (AuNP-NH2-lipase) was determined by various techniques such as agarose gel electrophoresis, zeta measurement, FTIR-spectroscopy and TEM. Further, catalytic parameters (Vmax, KM,app, Kcat, and Kcat/KM,app) have been studied to establish activity enhancement upon immobilization. The data also suggested that, AuNP-NH2-lipase has desirable improved parameters such as temperature and storage stability. The thermodynamic parameters for the kinetics of deactivation (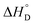 ,
, 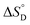 and
and 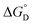 ) of the AuNP-NH2-lipase and free lipase demonstrated better stability of the conjugate. CD and fluorescence spectroscopic studies revealed minor structural rearrangements in the enzyme upon conjugation. Thus the AuNP-NH2-lipase conjugate represents a novel enzyme preparation with attributes of high activity and stability that could be an attractive choice in diverse applications ranging from catalysis to diagnostics.
) of the AuNP-NH2-lipase and free lipase demonstrated better stability of the conjugate. CD and fluorescence spectroscopic studies revealed minor structural rearrangements in the enzyme upon conjugation. Thus the AuNP-NH2-lipase conjugate represents a novel enzyme preparation with attributes of high activity and stability that could be an attractive choice in diverse applications ranging from catalysis to diagnostics.



 Please wait while we load your content...
Please wait while we load your content...