Cell responses on a H2Ti3O7 nanowire film
Abstract
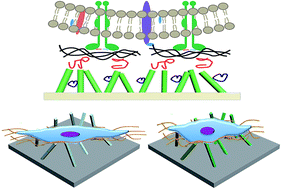
Alkali treatment has been widely used for the surface modification of Ti and Ti alloys for clinical applications. However, the mechanism underlying the effects of the alkali-treated Ti surface on the cell response still needs to be explored. In this study, we demonstrated that the cell responses on a H2Ti3O7 nanowire film, normally titanate generated on a Ti surface via alkali treatment and ion exchange, were sensitive to the surface hydroxyl groups of the H2Ti3O7 nanowire films, i.e. the total amount as well as comparative ratio of the distinct type of surface hydroxyl groups (bridging-OH and terminal-OH). The surface hydroxyl groups of H2Ti3O7 nanowires were further controlled via heat treatment to produce anatase TiO2 nanowire films. Although there was no difference in both topographies, cells on a H2Ti3O7 nanowire film showed more elongated shape than those on an anatase nanowire film. Moreover, the expression of ALP of cells on H2Ti3O7 upregulated as compared to that on the anatase nanowire film at day 4, suggesting enhanced osteogenic capacity at an early stage. These results could be attributed to the difference in the distinct ratio of the terminal hydroxyl groups to the bridging hydroxyl groups, which was 0.42 for the H2Ti3O7 nanowire film and 0.75 for the anatase nanowire film. It appeared that the bridging hydroxyl groups on H2Ti3O7 were efficient in attracting Ca2+, which influenced the cell morphology and further upregulated the early differentiation.


 Please wait while we load your content...
Please wait while we load your content...