Acid-promoted metal-free protodeboronation of arylboronic acids†
Abstract
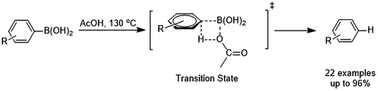
A facile acid-promoted protodeboronation of arylboronic acids in the absence of metal catalysts or any other additives is described. This protodeboronation is general for a range of arylboronic acids with both electron-donating and electron-withdrawing groups in good to excellent yields under air atmosphere. Density functional theory mechanistic studies showed that the protodeboronation of arylboronic acids followed an intermolecular metathesis via a four-membered ring transition state. The effect of the substituent of arylboronic acids in protodeboronation is also theoretically studied.


 Please wait while we load your content...
Please wait while we load your content...