Norsampsone E, an unprecedented decarbonyl polycyclic polyprenylated acylphloroglucinol with a homoadamantyl core from Hypericum sampsonii†
Abstract
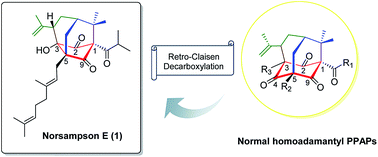
Norsampsone E (1), an unprecedented decarbonyl polycyclic polyprenylated acylphloroglucinol, together with one new and two known analogues (2–4), was isolated from the aerial parts of Hypericum sampsonii. Compound 1 featured an unusual homoadamantyl skeleton with the loss of C-4 carbonyl and followed by the formation of a new C–C bond between C-3 and C-5. Their structures and absolute configurations were elucidated by extensive NMR spectroscopy, single-crystal X-ray experiments, ECD calculations and CD comparison methods, and further confirmed using the single-crystal X-ray structures of biogenetically related congeners 3 and 4. Besides, compounds 1 and 2 showed direct binding to the RXRα-LBD protein in the SPR assay with a KD of 10.28 μM and 31.70 μM, respectively. Furthermore, compound 1 (5–20 μM) showed potent RXRα transcriptional inhibitory activity in a dose dependent manner, while compound 2 (5–20 μM) slightly enhanced RXRα transactivation.


 Please wait while we load your content...
Please wait while we load your content...