Catalytic effect of MnFe2O4 on dehydrogenation kinetics of NaAlH4–MgH2
Abstract
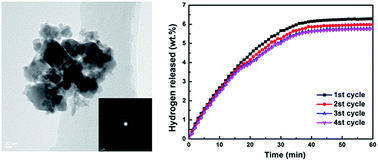
The hydrogen desorption properties and decomposition processes of MnFe2O4-doped NaAlH4–MgH2 composites are investigated. The onset temperatures of three dehydrogenation steps of MnFe2O4-doped NaAlH4–MgH2 composites are 92, 116 and 115 °C lower than those of as-milled NaAlH4–MgH2. Furthermore, NaAlH4–MgH2 + 3 mol% MnFe2O4 displays good cycling stability at 300 °C and only a slight capacity loss after four cycles. The apparent activation energies estimated from the Kissinger analysis for NaAlH4- and MgH2-relevant decomposition are found to be 51.29 and 105.34 kJ mol−1, respectively. XRD and XPS results show that NaFeO2, Mg2MnO4 and the amorphous Mn/MnOx species as well as iron oxides contribute to enhancing the dehydrogenation performance of NaAlH4–MgH2.


 Please wait while we load your content...
Please wait while we load your content...