Mn/SAPO-34 as an efficient catalyst for the low-temperature selective catalytic reduction of NOx with NH3
Abstract
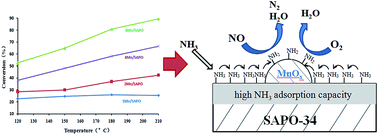
A series of Mn-exchanged SAPO-34 catalysts were synthesized and developed as catalysts for the low temperature selective catalytic reduction (SCR) of NO with ammonia in the presence of excess oxygen. 4Mn/SAPO-34 showed the highest SCR activity in the temperature range of 120–210 °C. The microstructure of zeolite supports, acidity, manganese species and reaction mechanism were investigated in detail by BET, NH3-TPD, UV-Vis, H2-TPR, XPS and in situ DRIFTS. Mn3+ and Mn4+ related species were proved to be the active sites for the SCR reaction. The Eley–Rideal mechanism was proved to be effective on 4Mn/SAPO-34 for the low temperature SCR reaction in which coordinated NH4+ species react with gas-phase NO to form an activated transition state and subsequently decomposed to N2 and H2O.


 Please wait while we load your content...
Please wait while we load your content...