Magnetorefrigeration capability of a gadolinium(iii) coordination polymer containing trimesic acid: a correlation between the isothermal magnetic entropy change and the gadolinium content
Abstract
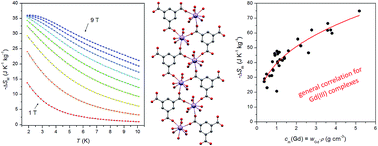
The Gd(III) coordination polymer [Gd(mes)(H2O)6]n (1), where H3mes stands for trimesic acid, was subjected to variable temperature/field magnetizations and heat capacity measurements. Analyses of the magnetic data revealed negligible magnetic interactions (zJ = −0.39 cm−1) and the isothermal magnetic entropy change −ΔSM = 36.0 J K−1 kg−1 at T = 2 K and B = 9 T. The evaluation of the heat capacity data resulted in a similar value of −ΔSM = 34.8 J K−1 kg−1. Thus, compound 1 shows a quite large magnetocaloric effect among Gd(III)-based molecular coolants. Furthermore, the correlation of the magnetocaloric effect based on the mass concentration of Gd atoms (cm(Gd)) was proposed as −ΔSM = 39.5cm(Gd)0.364 taking into account the experimental data for more than thirty Gd(III) compounds.


 Please wait while we load your content...
Please wait while we load your content...