Mechanistic investigations and molecular properties of 1,2-bis(ferrocenyl)dimetallenes including group 14 elements†
Abstract
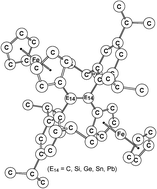
The molecular properties and reactive activities of the d-π conjugated (E)-Tip(Fc)E14![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) E14(Fc)Tip (1-C, 1-Si, 1-Ge, 1-Sn, and 1-Pb) systems possessing the E14
E14(Fc)Tip (1-C, 1-Si, 1-Ge, 1-Sn, and 1-Pb) systems possessing the E14![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) E14 unit were explored using density functional theory (M06-2X/Def2-SVPD). The theoretical investigations on the basis of several physical properties (the bonding analysis, UV-vis and Raman spectra) indicate that a coupling occurs between the double bonded moiety, E14
E14 unit were explored using density functional theory (M06-2X/Def2-SVPD). The theoretical investigations on the basis of several physical properties (the bonding analysis, UV-vis and Raman spectra) indicate that a coupling occurs between the double bonded moiety, E14![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) E14, and the ferrocenyl groups. Moreover, the present theoretical findings strongly suggest that the chemical reactivity of such d-π conjugated molecules increase in the order as follows: 1-C ≪ 1-Si < 1-Ge < 1-Sn < 1-Pb. Specifically, the larger the atomic radius of the group 14 element involved in such d-π conjugated compounds, the smaller its π bond strength and more facile its [1 + 4] or [1 + 2] cycloaddition with either a butadiene or a selenium atom, respectively. The singlet–triplet energy splittings, based on the VBSCD model, have been used as a guide to interpret the reactivity.
E14, and the ferrocenyl groups. Moreover, the present theoretical findings strongly suggest that the chemical reactivity of such d-π conjugated molecules increase in the order as follows: 1-C ≪ 1-Si < 1-Ge < 1-Sn < 1-Pb. Specifically, the larger the atomic radius of the group 14 element involved in such d-π conjugated compounds, the smaller its π bond strength and more facile its [1 + 4] or [1 + 2] cycloaddition with either a butadiene or a selenium atom, respectively. The singlet–triplet energy splittings, based on the VBSCD model, have been used as a guide to interpret the reactivity.


 Please wait while we load your content...
Please wait while we load your content...