Regio- and stereoselective syntheses of allylic thioethers under metal free conditions†
Abstract
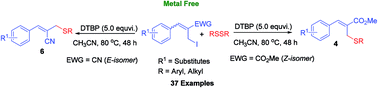
A metal free, regio and stereoselective syntheses of allylic thioethers using allyl iodides and aryl or alkyl disulfides as coupling partners is described. The densely functionalized allyl iodides having different stereochemistry (E & Z) reacted well with a variety of disulfides in a regio and stereoselective manner providing the resulting allyl aryl thioethers in 62–92% yields.


 Please wait while we load your content...
Please wait while we load your content...