Dual role of polyphosphoric acid-activated nitroalkanes in oxidative peri-annulations: efficient synthesis of 1,3,6,8-tetraazapyrenes†
Abstract
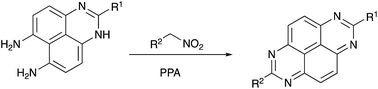
A highly efficient synthetic method for expeditious and selective assembly of tetraazopyrenes (TAPy) is reported, based on the novel reaction of electrophilically activated nitroalkanes with aromatic amines. Remarkably, the nitroalkanes play a dual role in this process, also serving as mild and efficient oxidants promoting aromatization of the final product and allowing for the exclusion of a poorly controllable aerobic treatment.


 Please wait while we load your content...
Please wait while we load your content...