Theoretical study on the photooxygenation and photorearrangement reactions of 3-hydroxyflavone†
Abstract
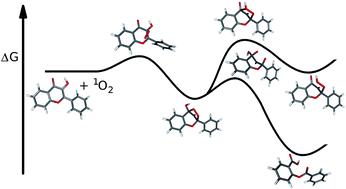
The photooxygenation of 3-hydroxyflavone (3HF) into O-benzoyl salicylic acid and the photorearrangement of 3HF into 3-hydroxy-3-phenyl-indane-1,2-dione have been studied using theoretical calculations. These are the main photodegradation reactions of this versatile fluorescent probe which exhibits excited state intramolecular proton transfer (ESIPT). The Gibbs free energies for the ground/excited state species were computed at the DFT/TD-DFT level of theory. The calculations on the direct photooxygenation (T1 state phototautomeric form of 3HF + 3O2) as well as on the photosensitized reaction (S0 state normal form of 3HF + 1O2) confirmed the feasibility of the reaction routes via an endoperoxide intermediate. In contrast, the alternative mechanism, via an exoperoxide intermediate proved kinetically inaccessible. In the calculation of the rearrangement reaction, the T1 state phototautomeric form was considered the reacting species (as indicated by photochemical experiments) and the free energy profile was calculated through T1 stationary points. The results suggested the occurrence of an intermediate with a 1-indanone skeleton, formed through a transition state with a relatively high free energy barrier corresponding to the folding of the pyrone ring.


 Please wait while we load your content...
Please wait while we load your content...