Concentration-dependent self-assembly structures of an amphiphilic perylene diimide with tri(ethylene glycol) substituents at bay positions†
Abstract
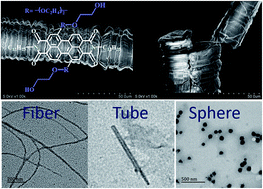
In this work, an amphiphilic perylene diimide (1,7-TEG-PDI-C12) that bears two hydrophilic triethylene glycol (TEG) chains at its bay position, and two hydrophobic dodecyl chains at its imide position was synthesized to identify the roles of concentration and H-bonding on the self-assembly morphology of the amphiphilic PDI. Since 1,7-TEG-PDI-C12 was prepared from the reaction of two bifunctional reactants, TEG and N,N′-bis(n-dodecyl)-1,7-dibromo-perylene-3,4:9,10-tetracarboxylic diimide, careful choices of solvent, base, and the stoichiometry of crown ether and base were found to be critical in reaching high reaction yield under mild conditions. TEM and SEM results revealed the abundant concentration-dependent self-assembly morphologies of 1,7-TEG-PDI-C12. Characterization results including UV-vis, fluorescence, NMR, IR and XRD analysis show that the formation of the self-assembled structure is a synergetic result of the intermolecular π–π interaction and H-bonding of 1,7-TEG-PDI-C12.


 Please wait while we load your content...
Please wait while we load your content...