Oxygen-vacancy-promoted catalytic wet air oxidation of phenol from MnOx–CeO2†
Abstract
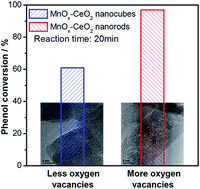
Catalytic oxidation can be effectively promoted by the presence of oxygen vacancies on the catalyst surface. In this study, the effect of oxygen vacancies on the catalytic wet air oxidation (CWAO) of phenol was investigated with CeO2 and MnOx–CeO2 as catalysts. CeO2 and MnOx–CeO2 catalysts with different amounts of oxygen vacancies were obtained via hydrothermal methods and applied for the CWAO of phenol. It was found that CeO2 and MnOx–CeO2 nanorods were much more active than the cubic nanorods. The physicochemical properties of the samples were characterized by TEM, XRD, BET, XPS, and H2-TPR techniques. The results revealed that the presence of oxygen vacancies in CeO2 and MnOx–CeO2 catalysts could increase the oxidizing ability of the catalysts surface. The addition of Mn could greatly improve the adsorption ability of CeO2 and more efficiently oxidize phenol and its intermediates. The synergy between Mn and Ce could further improve the catalyst redox properties and produce a larger amount of active oxygen species, which is the reason why MnOx–CeO2 nanorods are the most active catalysts among the catalysts investigated in this study.


 Please wait while we load your content...
Please wait while we load your content...