Selective C–H bond hydroxylation of cyclohexanes in water by supramolecular control†
Abstract
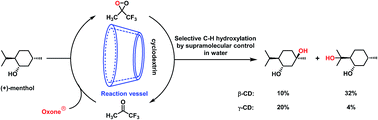
A new approach for selective hydroxylation of non-activated cyclohexanes using dioxirane generated in situ in water through supramolecular control has been developed. Using β-CD and γ-CD as the supramolecular hosts, selective hydroxylation of cyclohexane substrates, including trans/cis-1,4-, 1,3- and 1,2-dimethylcyclohexanes and trans/cis-decahydronaphthalene, was achieved in up to 54% yield in water. Furthermore, site-selective C–H bond hydroxylation of (+)-menthol was achieved by obstructing the approach of dioxirane to the C–H bond with higher steric hindrance through inclusion complexation with β-CD and γ-CD in water.


 Please wait while we load your content...
Please wait while we load your content...