Degradation of azo dye with activated peroxygens: when zero-valent iron meets chloride†
Abstract
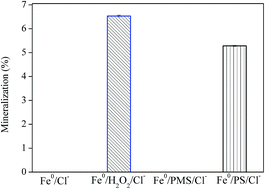
Degradation of acid orange 7 (AO7) by Fe0-based Advance Oxidation Process (AOPs) with common peroxygens, persulfate (PS), peroxymonosulfate (PMS) and hydrogen peroxide (H2O2), was investigated, in which sulfate radicals (SO4˙−) and/or hydroxyl radicals (˙OH) are powerful oxidizing species. The effects of Fe0 dosage, peroxygen concentration, initial pH and the presence of chloride on the degradation of AO7 were examined. The AO7 degradation efficiencies by four systems, including Fe0, Fe0/H2O2, Fe0/PMS and Fe0/PS were compared. AO7 degradation rate by Fe0 activated AOPs in descending order is H2O2 ≧ PS > PMS. Increasing acidity and iron dosage favored a rapid degradation of AO7. The presence of chloride greatly inhibited dye removal in Fe0/H2O2 and Fe0/PS systems, whilst accelerated dye degradation was observed in the Fe0/PMS system. In contrast, mineralization of AO7 in the Fe0/PMS/Cl− system was minimal, because of formation of lots of refractory chlorinated phenols as identified by GC-MS. These findings are useful for selecting the most appropriate technology for textile wastewater treatment, depending on the wastewater constituents and pH.


 Please wait while we load your content...
Please wait while we load your content...