Chromium nitride as a stable cathode current collector for all-solid-state thin film Li-ion batteries†
Abstract
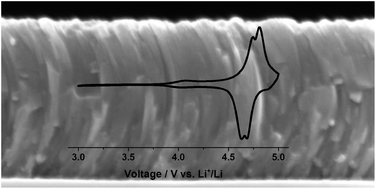
The development of highly oxidation resistant current collectors which are inert against lithium at elevated temperatures (>600 °C) and high potentials (3–5 V vs. Li+/Li) is required for the realization of high voltage, thin film, all-solid-state Li-ion batteries. This is due to the method of building such batteries using layer by layer deposition, requiring the first layer to remain stable during all subsequent processing steps. A new cathode current collector based on Cr2N thin films and prepared by reactive pulsed DC sputtering at 300 °C is reported here. By varying the nitrogen partial pressure in the reactor several CrxN thin film alloys are prepared and their microstructural and electrical properties are characterized. We demonstrate that the alloy with an estimated composition of Cr2.1N exhibits a relatively low sheet resistance of 2.6 Ω sq−1 for a ∼500 nm film, high oxidation resistance and no reaction with lithium up to 600 °C. Furthermore, we observed electrochemical stability in the potential range 3–5 V vs. Li+/Li. Finally, as a proof of concept the electrochemical behavior of cells using Cr2.1N thin films as a current collector for the high voltage cathode LiMn1.5Ni0.5O4 is presented.


 Please wait while we load your content...
Please wait while we load your content...