Ervaoffines E–G, three iboga-type alkaloids featuring ring C cleavage and rearrangement from Ervatamia officinalis†
Abstract
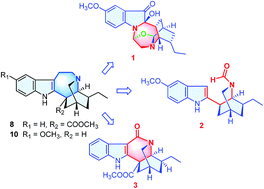
Ervaoffine E (1), a new pseudoindoxyl alkaloid possessing a unique rearranged 1,4-diazacycloheptane skeleton, ervaoffine F (2), the first 5,6-seco-6-nor iboga-type alkaloid featuring ring C cleavage, and ervaoffine G (3), bearing an unusual contracted valerolactam ring, together with six known alkaloids were isolated from Ervatamia officinalis. Their structures and absolute configurations were determined by extensive spectroscopic analysis, single-crystal X-ray diffraction and quantum chemical ECD calculations. Plausible biogenetic pathways of these new alkaloids were also proposed and provided new insights into the structural plasticity of ring C in iboga-type alkaloids. Compound 3 exhibited a significant neuroprotective effect against oxygen-glucose deprivation (OGD)-induced damage of cultured cortical neurons, an in vitro model of ischemic stroke.


 Please wait while we load your content...
Please wait while we load your content...