A review of Mn-containing oxide catalysts for low temperature selective catalytic reduction of NOx with NH3: reaction mechanism and catalyst deactivation
Abstract
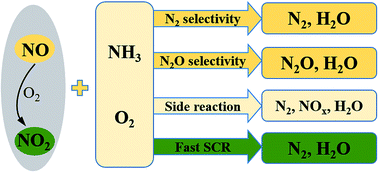
Atmospheric pollutants of nitrogen oxides (NOx) can be reduced by selective catalytic reduction (SCR). SCR of NOx with ammonia (NH3) at low temperatures has attracted much interest for high nitric oxide (NO) conversion, and this method is dominated by catalysts. Manganese (Mn)-containing oxide catalysts exhibit high activity and selectivity for the unique redox property of manganese oxides (MnOx). The reaction mechanisms and deactivation processes are summarized in this review. SCR of NOx with NH3 follows both the Langmuir–Hinshelwood and the Eley–Rideal mechanisms, which also contribute to the nitrous oxide formation. Fast SCR has a higher reaction rate than standard SCR. Mn-containing catalysts could also be deactivated by sulfur oxides and water vapor. The deactivation process of sulfur dioxide can be classified into two categories: deposition of (NH4)2SO4 and sulfation of active sites. The deactivation caused by water vapor can be attributed to the competitive adsorption. The adsorption of water on catalysts' surface blocked the active sites, which are provided for the adsorption of NH3 and NO. Alkali, alkaline earth and heavy metal ions existing in fine fly ash can also damage the catalysts' acid sites. A notable improvement on performance was obtained when Mn-containing catalysts were doped with a transition metal, for these enhanced its adsorption capacity and oxidation ability. Furthermore, this review gives a comprehensive discussion of the synergistic mechanism between bi-metal or multi-metal oxides. Major conclusions and several possible directions for further research are presented finally.
- This article is part of the themed collection: 2017 Review articles


 Please wait while we load your content...
Please wait while we load your content...